Pediatric cancer: Understanding disease to improve lives

Pediatric cancer, defined as cancer in children and adolescents under 18, is rare. In Norway, it accounts for less than 1% of all cancer cases, with approximately 200 new diagnoses annually. Pediatric cancer includes several main diagnostic groups, each with multiple subtypes, meaning that few children are affected by each specific diagnosis. The most common childhood cancers are leukaemia, lymphoma, and tumours of the central nervous system (brain and spinal cord). Other cancers are classified as solid tumours outside the central nervous system, a broad category that includes bone and soft tissue tumours.
This project focuses on leukaemia and selected solid tumours, including bone and soft tissue tumours, as defined by the World Health Organisation’s current classification. Cancer in children is biologically distinct from cancer in adults, primarily because children’s bodies are still growing and developing. While adult cancers often arise due to accumulated genetic damage over many years, childhood cancers typically originate from stem or progenitor cells, driven by fewer genetic changes, and usually occur without apparent environmental or lifestyle-related causes.
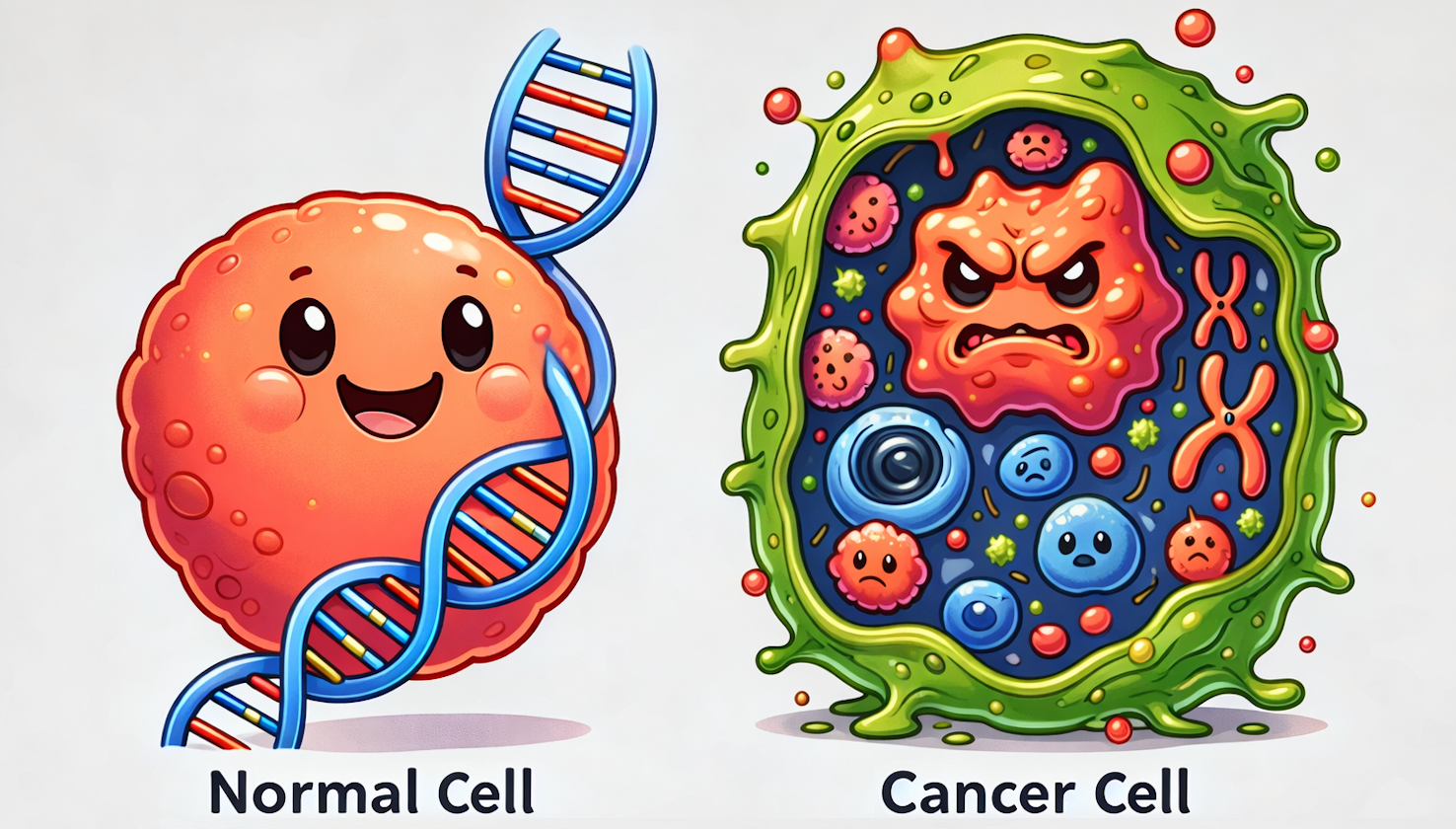
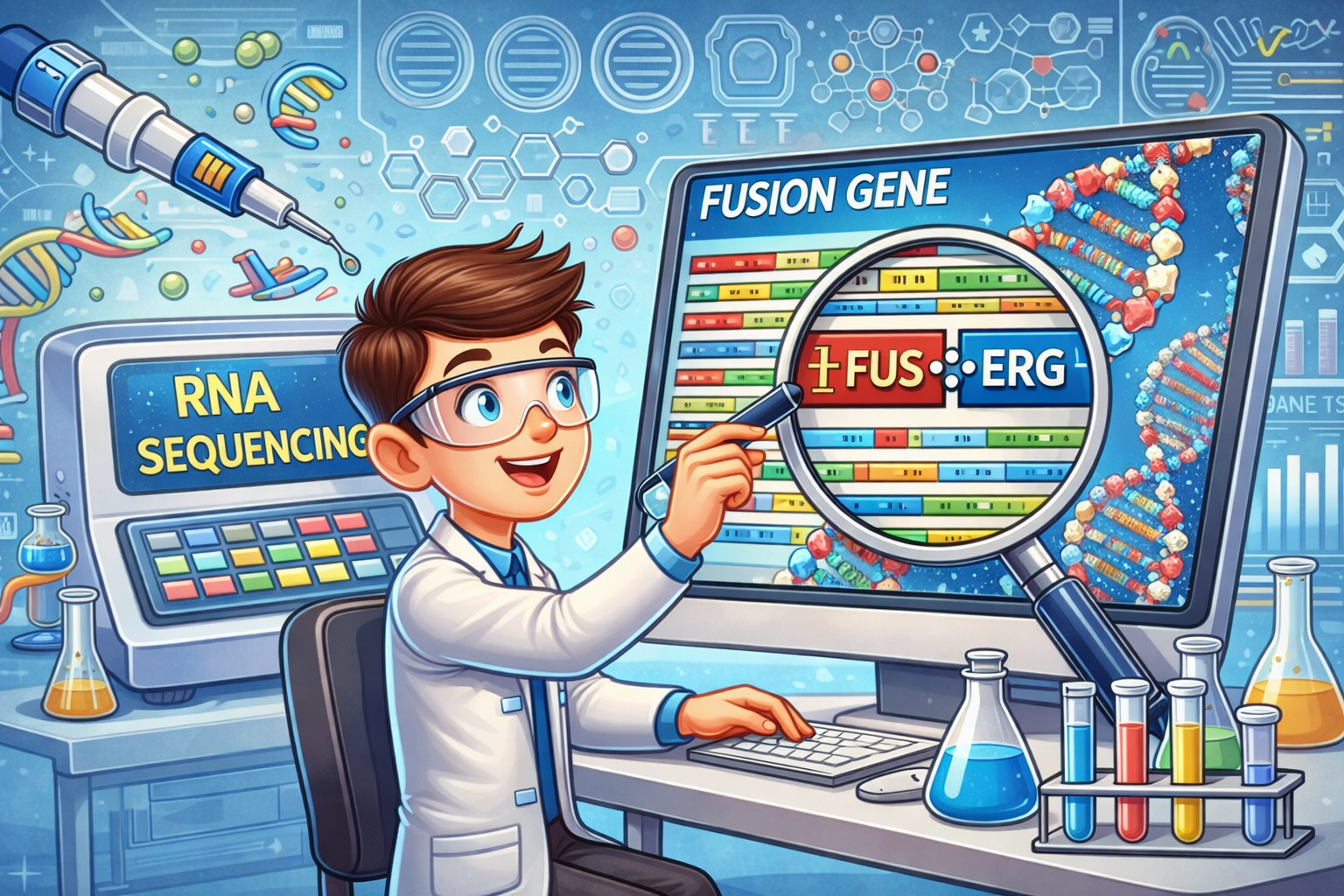
At the molecular level, pediatric cancers show a lower mutational burden, greater epigenetic dysregulation, and a higher prevalence of fusion genes compared to adult cancers. Fusion genes, which form when parts of two different genes merge to create a hybrid gene, can alter normal cellular function. These molecular features affect how cells grow, divide, and mature. In healthy cells, genetic material is well organized and tightly regulated. However, in cancer cells, this organization is disrupted. Changes in DNA, such as the formation of fusion genes, can interfere with essential cellular processes and drive uncontrolled cell growth. Such genetic alterations often crucially impact childhood cancer’s development and response to treatment.
Thanks to major advances in medical care, survival rates for many childhood cancers have improved significantly. However, treatment can be demanding and result in long-term side effects impacting quality of life. Because childhood cancer has distinct biological characteristics, there is a strong need for knowledge that informs diagnosis and treatment tailored specifically for children.
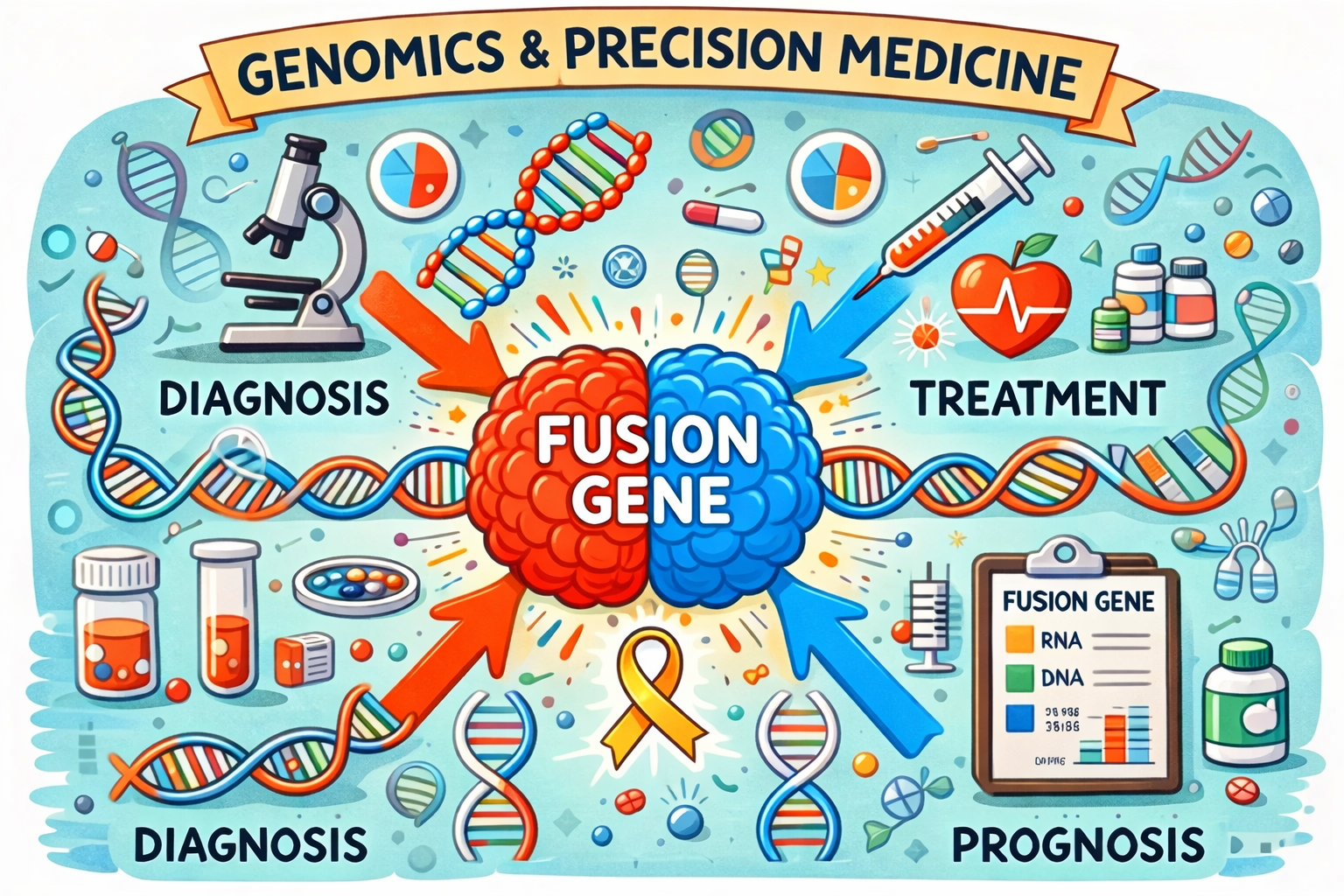
Our project aims to improve understanding of the genetic changes underlying pediatric cancer by using RNA sequencing as the primary high-throughput approach to identify fusion genes and other cancer-driving genetic alterations. By studying tumour tissue at the molecular level, we seek to refine diagnosis, improve disease classification accuracy, and contribute to developing more precise and less invasive treatment strategies. Ultimately, our goal is to support precision diagnostics, ensuring every child receives the correct diagnosis and the optimal treatment as early as possible.
|

|
|

|
|
|
